Delving into the realm of chemistry, we embark on a journey to explore the intricacies of naming mixed ionic and covalent compounds. These compounds, characterized by their unique blend of ionic and covalent bonds, present a fascinating challenge that requires a deep understanding of chemical principles.
As we unravel the IUPAC rules and delve into the factors influencing bond type, we will gain a comprehensive grasp of the systematic approach to naming these intriguing compounds.
Throughout this discourse, we will encounter practical applications and delve into real-world examples, solidifying our understanding of the significance of mixed ionic and covalent compounds in various industries. By the conclusion of this exploration, we will emerge with a profound appreciation for the intricacies of chemical nomenclature and its role in unraveling the complexities of the molecular world.
Understanding Mixed Ionic and Covalent Compounds
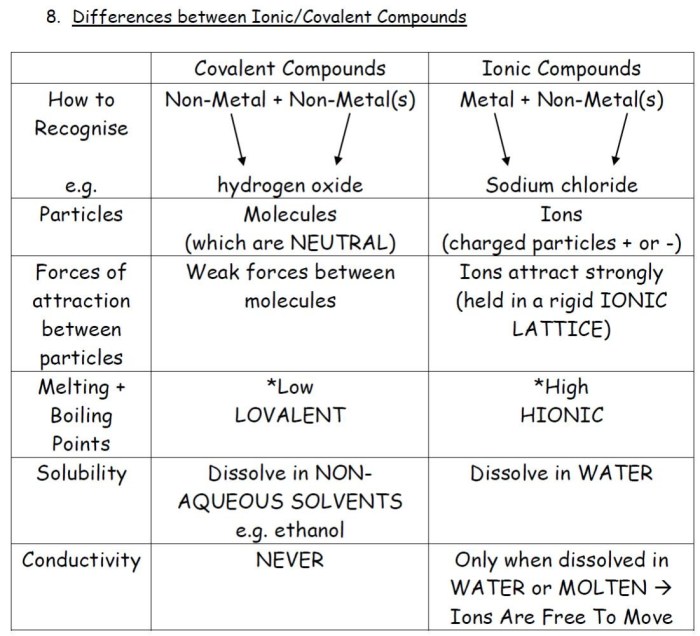
Mixed ionic and covalent compounds exhibit characteristics of both ionic and covalent bonds. Ionic bonds arise from the electrostatic attraction between ions, while covalent bonds involve the sharing of electron pairs. These compounds possess properties that are intermediate between pure ionic and pure covalent compounds.
Mixed ionic and covalent compounds typically contain a metal cation and a non-metal anion. The metal atom loses one or more electrons to achieve a stable octet configuration, forming a positively charged cation. The non-metal atom gains the lost electrons, forming a negatively charged anion.
The electrostatic attraction between the cation and anion results in an ionic bond.
However, due to the difference in electronegativity between the metal and non-metal atoms, the electron pair shared between them is not equally distributed. The more electronegative non-metal atom attracts the electron pair more strongly, resulting in a partial negative charge on the anion and a partial positive charge on the cation.
This partial charge separation introduces a degree of covalent character to the bond.
The extent of covalent character in a mixed ionic and covalent compound depends on several factors, including the electronegativity difference between the metal and non-metal atoms, the size of the ions, and the coordination number of the metal ion.
Factors Influencing Bond Type
The following table summarizes the factors that influence the type of bond formed between atoms:
| Factor | Effect on Bond Type |
|---|---|
| Electronegativity difference | Greater difference leads to more ionic character |
| Size of ions | Larger ions lead to more covalent character |
| Coordination number | Higher coordination number leads to more covalent character |
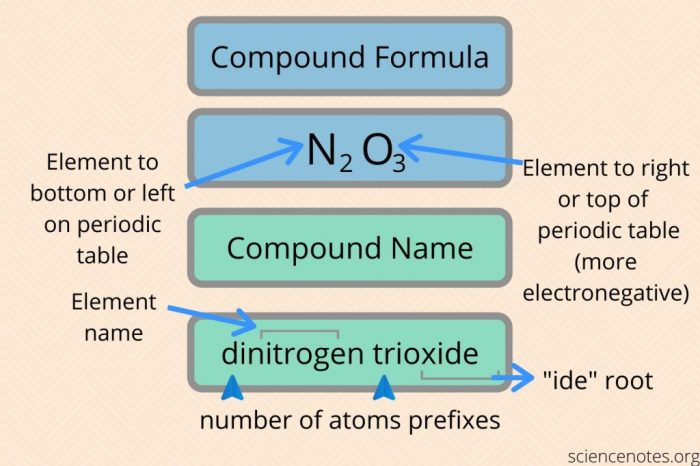
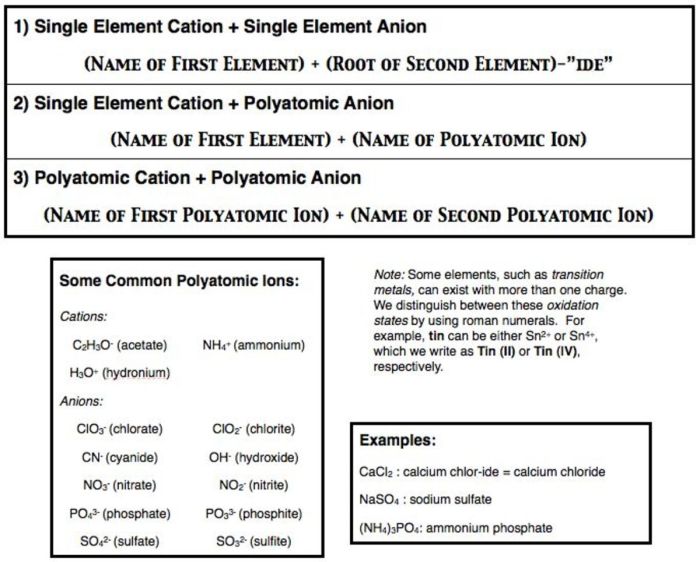
Naming Mixed Ionic and Covalent Compounds
The IUPAC rules for naming mixed ionic and covalent compounds follow a systematic approach:
- The cation is named first, using the Roman numeral to indicate its charge if it is a variable charge metal.
- The anion is named second, using the suffix “-ide”.
- The name of the compound is written as one word.
For example, the compound containing the cation Fe 2+and the anion O 2-would be named iron(II) oxide.
Top FAQs: Naming Mixed Ionic And Covalent Compounds
What is the difference between an ionic and a covalent bond?
Ionic bonds involve the transfer of electrons between atoms, resulting in the formation of charged ions. Covalent bonds, on the other hand, arise from the sharing of electrons between atoms.
How do you determine the type of bond in a mixed ionic and covalent compound?
The electronegativity difference between the constituent atoms is a key factor in determining the bond type. A large electronegativity difference typically indicates an ionic bond, while a small difference suggests a covalent bond.
What are the IUPAC rules for naming mixed ionic and covalent compounds?
The IUPAC rules prioritize the naming of the cation first, followed by the anion. The cation is named using the Roman numeral of its oxidation state, while the anion is named using its root name and the suffix “-ide”.