A flask contains equal masses of f2 and cl2 – In a flask containing equal masses of F2 and Cl2, a captivating chemical landscape unfolds, revealing the interplay between molar mass, volume, pressure, density, and reactivity. This in-depth exploration delves into the fundamental properties and potential reactions of these volatile gases, providing a comprehensive understanding of their behavior.
The masses of F2 and Cl2, represented by their chemical symbols, are equal within the flask. Their molar masses, calculated using atomic masses, determine the number of moles present. The volume of the flask, assuming standard temperature and pressure (STP), establishes the relationship between moles, volume, and pressure.
Overview of Flask Contents: A Flask Contains Equal Masses Of F2 And Cl2

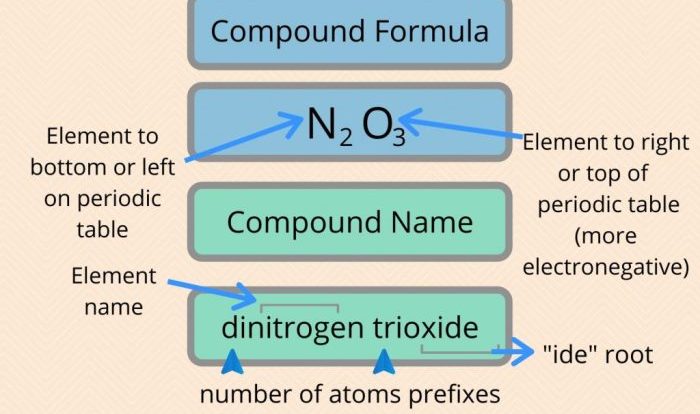
Consider a flask containing equal masses of F2 and Cl2. F2 and Cl2 are the chemical symbols for the elements fluorine and chlorine, respectively. In this scenario, the masses of F2 and Cl2 in the flask are identical.
Molar Mass and Moles
Molar mass refers to the mass of one mole of a substance. For F2, the molar mass is 38.00 g/mol (2 x 19.00 g/mol), and for Cl2, it is 70.90 g/mol (2 x 35.45 g/mol). Given the equal masses of F2 and Cl2 in the flask, we can determine the number of moles of each gas present using their respective molar masses.
Volume and Pressure
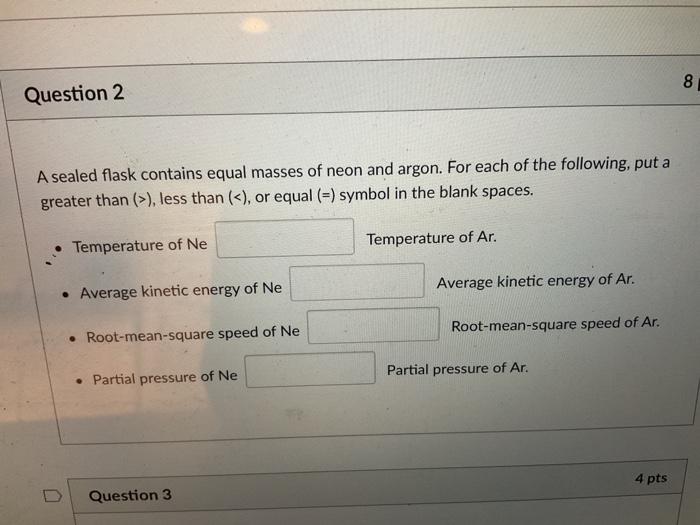
The number of moles of a gas is directly related to its volume and pressure. Assuming the flask is at standard temperature and pressure (STP), which is 0 °C (273.15 K) and 1 atm (101.325 kPa), we can calculate the volume of the flask using the ideal gas law: PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant (0.0821 L atm / (mol K)), and T is the temperature.
If the temperature or pressure were to vary, the volume of the flask would change accordingly. For example, increasing the temperature at constant pressure would result in an increase in volume, while increasing the pressure at constant temperature would lead to a decrease in volume.
Density and Mass

Density is defined as mass per unit volume. Using the mass and volume of the gas mixture in the flask, we can calculate its density. The density of the gas mixture would change if either the mass or volume were to vary.
For instance, adding more gas to the flask would increase its mass and, consequently, its density. Alternatively, increasing the volume of the flask while keeping the mass constant would result in a decrease in density.
Chemical Reactivity
Fluorine and chlorine are highly reactive elements. F2 is a pale yellow gas that reacts with most elements, including metals, non-metals, and even noble gases. Cl2 is a greenish-yellow gas that is also highly reactive, although less so than F2.
In the flask, F2 and Cl2 could potentially react to form various compounds, including interhalogens such as ClF, ClF3, and ClF5.
Due to the hazardous nature of these gases, appropriate safety precautions must be taken when handling them. These precautions include working in a well-ventilated area, wearing proper protective gear, and having adequate fire extinguishing equipment readily available.
Question Bank
What is the significance of equal masses of F2 and Cl2 in the flask?
Equal masses of F2 and Cl2 allow for direct comparison of their properties and potential reactions, providing insights into their behavior under identical conditions.
How does the molar mass of F2 and Cl2 affect their behavior?
Molar mass determines the number of moles present in the flask, which influences the volume and pressure of the gas mixture.
What safety precautions should be taken when handling F2 and Cl2?
F2 and Cl2 are highly reactive gases that require proper handling and storage to prevent accidents. Appropriate ventilation, protective gear, and adherence to safety protocols are essential.