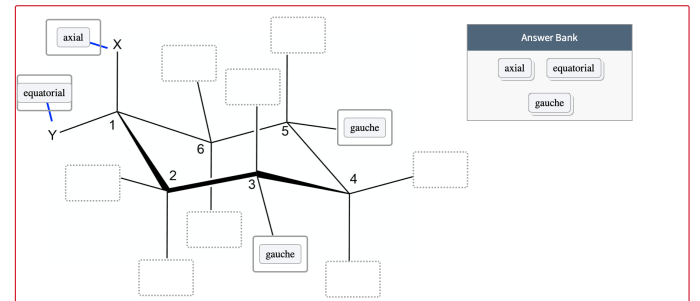
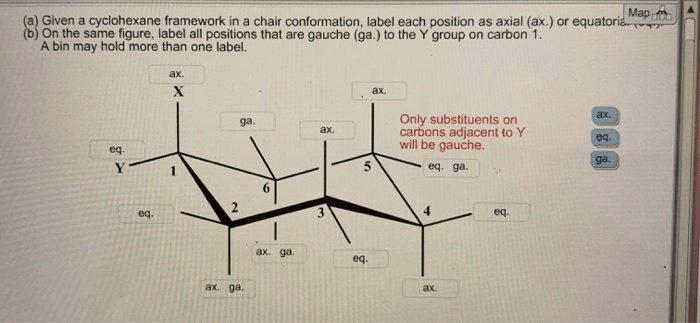
Consider the cyclohexane framework in a chair conformation, a topic that takes center stage in organic chemistry, invites us on an exploration of molecular structure and its profound influence on chemical behavior. This journey will delve into the spatial arrangement of carbon atoms, the concept of axial and equatorial hydrogen atoms, and the factors that govern the stability of the chair conformation.
Prepare to unravel the intricacies of cyclohexane’s conformational landscape, where substituents play a pivotal role in shaping its preferred orientation.
As we progress, we will uncover the significance of chair conformation in understanding chemical reactivity and selectivity, gaining insights into its applications in drug design and beyond. Brace yourself for a comprehensive examination of cyclohexane’s chair conformation, where knowledge and understanding converge to illuminate the molecular world.
Structural Features of Cyclohexane in Chair Conformation
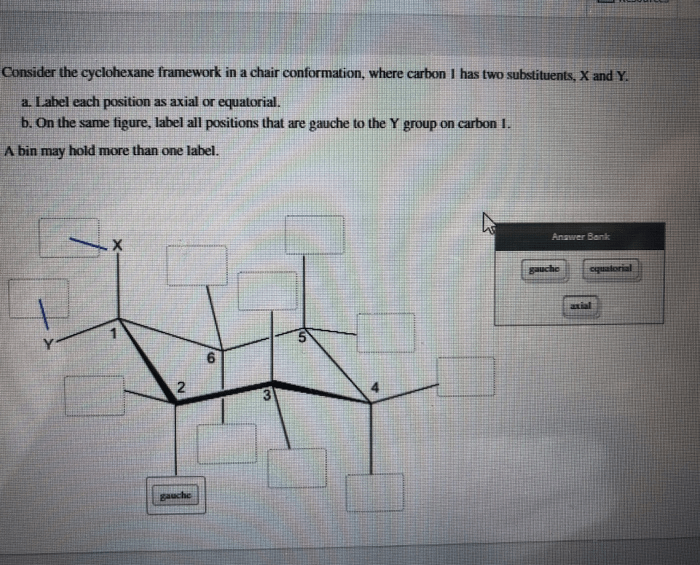
In the chair conformation of cyclohexane, the six carbon atoms are arranged in a puckered ring structure. Each carbon atom is bonded to two hydrogen atoms, one of which is positioned axially (above or below the ring) and the other equatorially (in the plane of the ring).
The chair conformation minimizes steric hindrance between the hydrogen atoms, resulting in a more stable arrangement. The axial hydrogen atoms are oriented perpendicular to the plane of the ring, while the equatorial hydrogen atoms are oriented parallel to the plane of the ring.
The chair conformation can be visualized using a Newman projection, which shows the ring from the side. In this projection, the axial hydrogen atoms are represented by lines pointing up or down, while the equatorial hydrogen atoms are represented by lines pointing left or right.
Conformational Stability of Cyclohexane
The chair conformation is the most stable conformation of cyclohexane due to several factors:
- Ring strain:The chair conformation minimizes ring strain, which is the distortion of the ring from its ideal geometry. In other conformations, such as the boat or twist-boat conformations, the ring is more distorted and therefore less stable.
- Steric interactions:The chair conformation minimizes steric interactions between the hydrogen atoms. In other conformations, the axial hydrogen atoms are closer together and experience more steric hindrance.
- Energy difference:The chair conformation is about 25 kJ/mol more stable than the boat conformation and 10 kJ/mol more stable than the twist-boat conformation.
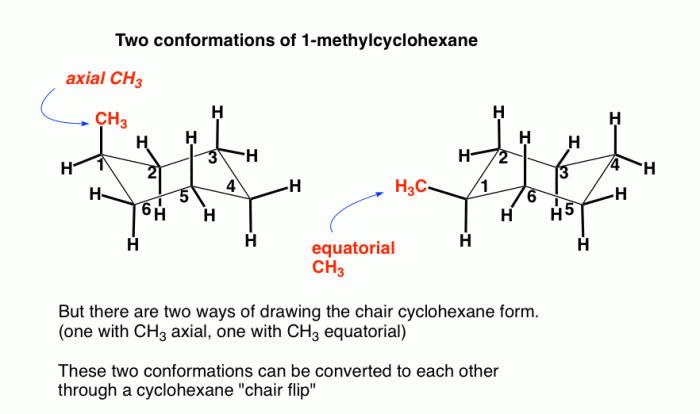
Substituent Effects on Chair Conformation
Substituents on the cyclohexane ring can influence the preferred chair conformation. Bulky or polar substituents can destabilize the chair conformation by introducing steric hindrance or electrostatic interactions.
For example, a bulky substituent in an axial position can experience steric hindrance with the hydrogen atoms on the adjacent carbon atoms. This can destabilize the chair conformation and favor the boat or twist-boat conformation.
Polar substituents can also influence the chair conformation through electrostatic interactions. For example, a polar substituent in an equatorial position can interact with the dipole moment of the ring, which can destabilize the chair conformation and favor the boat or twist-boat conformation.
Applications of Cyclohexane Chair Conformation
Understanding the chair conformation of cyclohexane is important in organic chemistry for several reasons:
- Chemical reactivity:The chair conformation can affect the chemical reactivity of cyclohexane. For example, electrophilic addition reactions prefer to occur on the equatorial face of the ring, while radical reactions prefer to occur on the axial face.
- Selectivity:The chair conformation can also affect the selectivity of reactions. For example, in the Diels-Alder reaction, the endo product is favored when the dienophile approaches the diene from the equatorial face of the ring.
- Drug design:The chair conformation of cyclohexane is also important in drug design. Many drugs contain cyclohexane rings, and the conformation of these rings can affect the drug’s activity and selectivity.
Q&A: Consider The Cyclohexane Framework In A Chair Conformation
What is the significance of the chair conformation in cyclohexane?
The chair conformation is the most stable conformation of cyclohexane due to its minimized ring strain and steric interactions. It plays a crucial role in determining the chemical reactivity and selectivity of cyclohexane derivatives.
How do substituents affect the chair conformation of cyclohexane?
Substituents can influence the chair conformation by introducing steric hindrance or electronic effects. Bulky substituents prefer equatorial positions to minimize 1,3-diaxial interactions, while electronegative substituents can stabilize axial positions through dipole-dipole interactions.
What are the applications of understanding chair conformation in organic chemistry?
Understanding chair conformation is essential for predicting the reactivity and selectivity of organic reactions involving cyclohexane derivatives. It finds applications in drug design, where the orientation of functional groups can impact biological activity, and in the synthesis of complex organic molecules.